
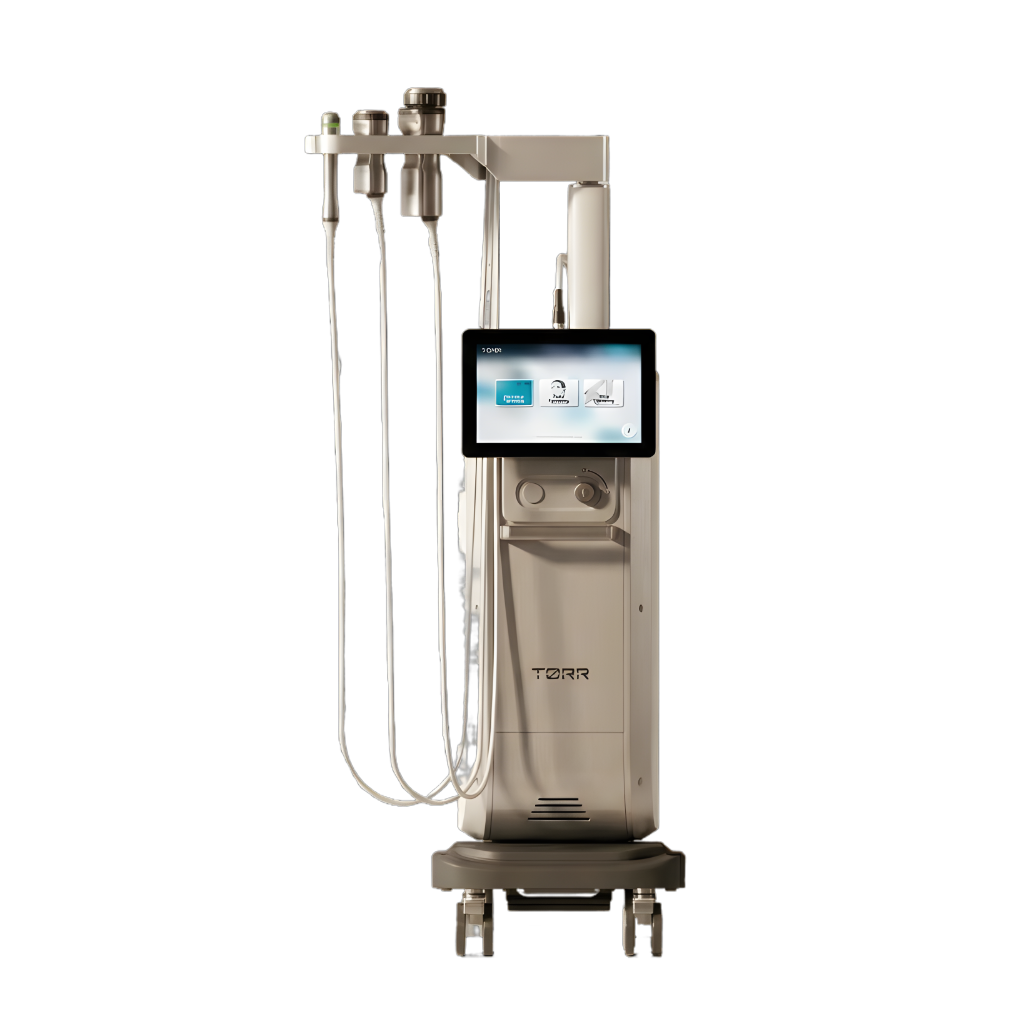
The New Standard

Automated RF workstation delivering precision, zero consumables, and consistent results



Automated RF workstation delivering precision, zero consumables, and consistent results
Traditional RF treatments rely heavily on operator skills. TORR RF solves this with patented Auto-Circular Motion technology.
Handpiece head automatically rotates at 68 rpm, creating a donut-shaped thermal field.
Automated motion ensures RF energy is distributed perfectly evenly, guaranteeing consistent results.
1 MHz Multi-Wave RF delivers stable thermal energy from dermis to subcutaneous fat.
FDA Cleared Applications
Mechanism
Delivers uniform thermal energy to deep dermis for collagen remodeling.
Benefit
Non-invasive facial contouring and skin tightening without downtime.
Mechanism
Deep thermal heating promotes vasodilation and metabolic activity.
Benefit
Temporary improvement of local blood circulation, revitalizing skin.
Mechanism
Heat targets subcutaneous fat with physical massage from rotating head.
Benefit
Temporary reduction in cellulite appearance, smoothing body lines.
Durability Meets Innovation
Intuitive 10.1-inch TFT LCD Touch Screen with preset memory.
Selectable Low / Deep Modes targeting depths from 1.5mm to 10mm.
3D vibration mitigates heat sensation for comfortable, pain-free experience.
Core Advantage: No consumable cartridges. Unlike HIFU or competitor RFs, our handpieces are durable and don't expire. You keep 100% of treatment revenue.
For Body Contouring & Cellulite (Auto-Rotation)
For Facial Tightening & Cheek Contouring
For Delicate Areas (Eyes/Periorbital)
Safety is our priority. Embedded sensors monitor skin temperature continuously. The Auto-Cutoff System immediately stops RF output if temperature exceeds set limits (30°C–50°C).
K212561
(Class II Medical Device)
Manufacturing License
No. 22-156
Manufactured in strictly
controlled facility
IEC 60601-1 &
IEC 60601-1-2
Sustainable Beauty based on Medical Science
Since 2017, BRITZMEDI has evolved from R&D institute to global manufacturer, creating "Standard-Premium" devices with high efficacy at rational prices.
Led by CEO Shin Jae Lee (Appointed Dec 2025), committed to transparency and global expansion.
FDA Registered Contract Manufacturer (Owner/Operator No: 10088936) ensuring full traceability.
We're looking for exclusive distributors who value clinical integrity and high ROI. Contact us for pricing and demo inquiries.
1211, 388, Dunchon-daero, Jungwon-gu,
Seongnam-si, Gyeonggi-do, Republic of Korea